Postoperative Pain Can Be Reduced by Using NOL Monitoring to Guide Analgesic Medication During Surgery-PR Newswire APAC
- Written by PR Newswire

|
RAMAT GAN, Israel, Sept. 29, 2020 /PRNewswire/ -- A new study has found that monitoring pain response levels during surgery with NOL technology (Medasense, Ramat Gan, Israel) can help reduce postoperative pain. Exploring the potential use of NOL monitoring to help enhance recovery after surgery, the study demonstrated that patient pain scores after surgery were 33% lower when administration of pain medication during surgery was guided with NOL monitoring.
"High pain scores following surgical procedures are common and are associated with poor patient outcomes; but on the other hand we know that excessive use of opioids administered during the surgery itself to prevent postoperative pain can cause other complications," explains Prof. Dahan of Leiden University Medical Center's Department of Anesthesiology, who led the study.
NOL monitoring provides a reliable index to objectively detect and quantify noxious stimuli during anaesthesia, when patients can't communicate, guiding the clinical team in tailored opioid dosing for each patient. Earlier studies have shown that the NOL index outperforms other indexes for monitoring of pain response to surgical stimuli and that NOL-guided analgesia resulted in reduced intraoperative opioid consumption, leading to fewer intraoperative hypotensive events.
The new study, just published in the British Journal of Anaesthesia (BJA)[1][1], followed 50 patients undergoing elective abdominal surgery in a two-center randomized controlled trial. The patients were randomly divided so that one group received NOL-guided analgesia dosing during the surgery and the control group received analgesics according to standard of care (based on hemodynamic monitoring). The study showed that while there was no increase in overall dosing in the NOL-guided group, the patients in that group reported less pain in the first 90 minutes compared to the control arm.
In addition, stress hormone levels (ACTH and cortisol) were on average up to 50% lower in the NOL-guided group, which clinically aligns with the lower pain response levels in those patients during surgery.
"Clinicians using NOL monitoring to guide analgesia are able to identify more accurately when a patient's pain response level rises and to tailor analgesic medication more accurately. As we found that opioid dosing overall was not different between the groups, we can confidently relate the difference in outcomes to the timing of individualized dosing as guided by the NOL monitor," Prof. Dahan concluded.
About Medasense and NOL Technology
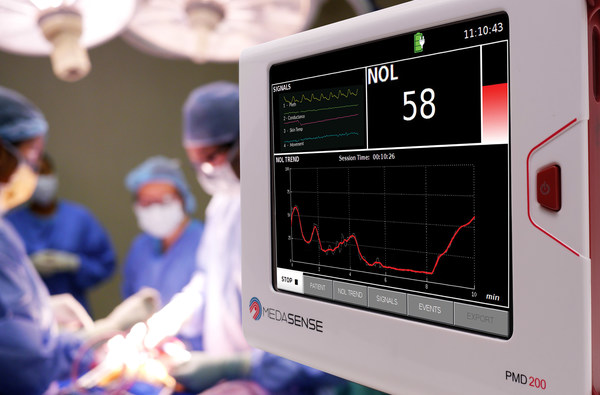
Medasense (www.medasense.com[2]) offers a breakthrough technology that enables clinicians to optimize and personalize pain control and avoid overmedication. Medasense's flagship product, the PMD-200™ with its NOL® index, is a unique platform that objectively monitors and quantifies the patient's pain response by means of artificial intelligence and a proprietary non-invasive sensor platform.
The PMD-200 is used to optimize pain management in critical care and operating rooms settings, where patients are unable to communicate.
Clinical studies have demonstrated its impact on patient safety and outcomes, including opioid sparing.
The PMD-200 is distributed in Europe exclusively by Medtronic, is cleared for marketing also in Canada, Latin America, Israel and Australia, and enables connectivity with Philips patient monitors.
Watch Medasense's 1-minute video[3]
1. Meijer, F., Honing, M., Roor, T., Toet, S., Calis, P., Olofsen, E., Martini, C., van Velzen, M., Aarts, L., Niesters, M., Boon, M., Dahan, A. (2020). Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. British Journal of Anaesthesia, In Press. DOI: https://doi.org/10.1016/j.bja.2020.07.057[4].
Photo - https://mma.prnasia.com/media2/1281101/medasense_pmd_200_nol_monitoring.jpg?p=medium600 Logo - https://mma.prnasia.com/media2/1169999/Medasense_Logo.jpg?p=medium600[5][6]
For further information please contact: Mira SoferVP Biz Dev & MarketingMedasense mira@medasense.com[7]
Source: Medasense Biometrics Ltd.
References
- ^ The new study, just published in the British Journal of Anaesthesia (BJA) (bjanaesthesia.org)
- ^ www.medasense.com (www.medasense.com)
- ^ Watch Medasense's 1-minute video (youtu.be)
- ^ https://doi.org/10.1016/j.bja.2020.07.057 (doi.org)
- ^ https://mma.prnasia.com/media2/1281101/medasense_pmd_200_nol_monitoring.jpg?p=medium600 (mma.prnasia.com)
- ^ https://mma.prnasia.com/media2/1169999/Medasense_Logo.jpg?p=medium600 (mma.prnasia.com)
- ^ mira@medasense.com (www.prnasia.com)
Read more https://www.prnasia.com/story/archive/3136329_AE36329_0